Abstract
Introduction: CD47, a cell-surface ligand overexpressed in various malignancies, binds to SIRPα on effector macrophages to promote tumor cell evasion of phagocytosis. Blockade of the CD47-SIRPα interaction provides a pro-phagocytic signal which enhances phagocytosis mediated by tumor-targeting antibodies such as rituximab. Agents targeting CD47 in combination with rituximab have demonstrated promising clinical activity in R/R NHL; however, the broad expression of CD47 leads to frequent on-target, off-tumor toxicities, including treatment-emergent hemolytic anemia. CC-95251 is a novel, fully human immunoglobulin G1 antibody that binds to SIRPα on monocytes and macrophages to potently block the CD47-SIRPα interaction. Here we report interim results from a phase 1 study evaluating CC-95251 combined with rituximab in patients (pts) with R/R NHL.
Methods: CC-95251-ST-001 (NCT03783403) is a multicenter, open-label, phase 1, dose-escalation and expansion study of CC-95251 in pts with advanced solid tumors and CD20+ R/R NHL. The primary objectives of the dose-escalation stage presented here are to evaluate the safety and tolerability of escalating doses of CC-95251 combined with rituximab and to define the maximum tolerated dose (MTD) and/or recommended phase 2 dose for the combination in pts with R/R NHL. Pts with CD20+ NHL who had progressed on standard anticancer therapy or for whom no approved conventional therapy was available were eligible for inclusion. Dose-escalation was conducted using an adaptive, 2-parameter Bayesian logistic regression model with overdose control. Pts were treated in 28-day cycles with CC-95251 administered intravenously at 3, 10, or 20 mg/kg every week (QW) and rituximab 375 mg/m 2 given on days 1, 8, 15, and 22 of cycle 1, on day 1 of cycles 2-5, and on day 1 of every other cycle from cycle 6 to 24 until disease progression or unacceptable toxicity.
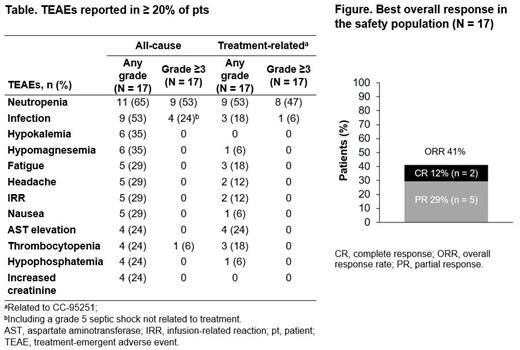
Results: As of April 30, 2021, 18 pts were enrolled and 17 had received ≥ 1 dose of CC-95251 and rituximab. Median age of the enrolled study population was 69 (range 30-84) years. Treated pts had received a median of 4 (range 1-7) prior systemic therapies, including 14/17 (82%) pts with confirmed prior rituximab exposure. Enrolled tumor types included R/R diffuse large B-cell lymphoma in 14 (78%) pts, follicular lymphoma in 2 (11%), and mantle cell lymphoma and marginal zone lymphoma in 1 (6%) pt each. Of the pts with available response data for prior lines of therapy (LOT), 7 (41%) had disease confirmed to be refractory to any prior LOT, including 5 (29%) refractory to rituximab-containing regimens, and 5 (29%) to their last LOT.
Pts received a median of 3 (range 1-12) cycles of CC-95251, with a median duration of treatment of 14.1 (range 1.1-47.3) weeks. There were no CC-95251 dose reductions. Five (29%) pts experienced ≥ 1 treatment-emergent adverse events (TEAEs) leading to CC-95251 dose interruption. To date, the MTD has not been reached for the combination of CC-95251 and rituximab. The most common (≥ 30%) TEAEs of any grade were neutropenia (11/17 [65%]), infections (9/17 [53%]), hypokalemia (6/17 [35%]), and hypomagnesemia (6/17 [35%]; Table). Grade ≥ 3 TEAEs reported in ≥ 2 pts were neutropenia (9/17 [53%]) and infections (4/17 [24%]). No treatment-related anemia, a notable toxicity of some anti-CD47 therapies, was reported with CC-95251 and there were no treatment-related deaths. The overall response rate was 41% (7/17), with 2/17 (12%) pts achieving a complete response (Figure). The median time to response was 7.6 weeks. Median duration of response has not been reached as responses are ongoing.
Pharmacokinetic (PK) analysis showed that CC-95251 exhibited dose-proportional increases in exposure at doses > 3 mg/kg QW indicating saturation of nonlinear clearance pathways or target saturation. Full receptor occupancy on peripheral monocytes was achieved at doses > 3 mg/kg QW. The estimated terminal half-life when in the linear range of clearance was approximately 12 days. No substantial differences in PK were observed at the given dose levels for CC-95251 monotherapy versus CC-95251 combined with rituximab.
Conclusions: CC-95251, a novel anti-SIRPα antibody, demonstrated a manageable safety profile and promising efficacy in combination with rituximab in pts with heavily pretreated CD20+ R/R NHL. The study continues to enroll in the dose-expansion phase. Updated safety and efficacy data will be presented.
Strati: Astrazeneca-Acerta: Research Funding; Roche-Genentech: Consultancy. Hawkes: Regeneron: Speakers Bureau; Specialised Therapeutics: Consultancy; Bristol Myers Squib/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck KgA: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel and accommodation expenses, Research Funding, Speakers Bureau; Janssen: Speakers Bureau; Antigene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Merck Sharpe Dohme: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees. Ghosh: Karyopharma: Consultancy, Honoraria; Genmab: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Adaptive Biotech: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; Genentech: Research Funding; AbbVie: Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; ADC Therapeutics: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Epizyme: Honoraria, Speakers Bureau. Tuscano: BMS, Seattle Genetics, Takeda, Achrotech, Genentech, Pharmacyclics, Abbvie: Research Funding. Chu: Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; BI: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Eli Lilly: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Merck KgaA: Other: DSMB. Patel: Bristol Myers Squibb: Current Employment. Burgess: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Hege: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Chhagan: Bristol Myers Squibb: Current Employment. Boyanapalli: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Day: Tracey Day: Current Employment. Shen: Bristol Myers Squibb: Current Employment. Mehta: Affirmed; Kite/Gilead; Roche-Genetech; Celgene/BMS; Oncotartis; Innate Pharmaceuticals; Seattle Genetics; Incyte; Takeda; Fortyseven Inc/Gilead; TG Therapeutics; Merck; Juno Pharmaceuticals/Bristol Myers Squibb: Research Funding; Seattle Genetics; Incyte; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics; Incyte; TG Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal